WHO Model List of Essential In Vitro Diagnostics
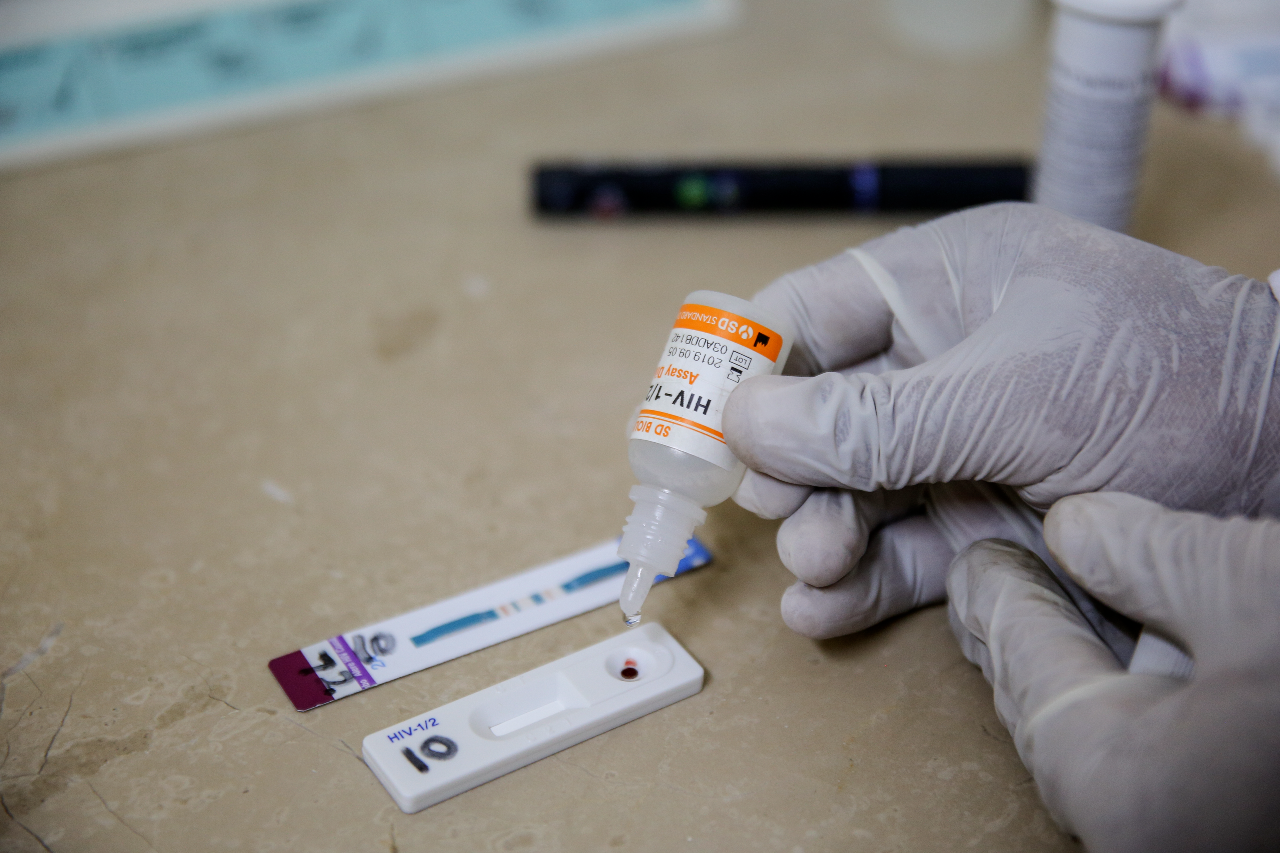
Access to good quality, affordable, and appropriate health products is indispensable to advance universal health coverage, address health emergencies, and promote healthier populations. Without access to In vitro diagnostics (IVDs), health providers cannot diagnose patients effectively and promptly or provide appropriate treatments.
The WHO Essential Diagnostic List (EDL) aims to provide evidence-based guidance and set a reference for the development or update of national lists of essential in vitro diagnostic tests. National essential medicines lists have been successful facilitating access to affordable medicines, particularly in low-resourced countries. It is expected that national essential diagnostics lists will provide similar benefits and improve access to essential in vitro diagnostic tests. It will also contribute towards health systems strengthening and realizing universal health coverage, which is central to Goal 3 of the Sustainable Development Goals (Ensure healthy lives and promote well-being for all at all ages).



